Medtronic's latest iteration of its Activa neurostimulator is not just a therapy device, but a brain activity information recorder that could provide insights into such devastating neurological disorders as Parkinson's disease.
The first two implants of the Activa PC+S DBS system in the U.S. recently took place at Stanford Hospital & Clinics and the UC San Francisco (UCSF) Medical Center in patients with advanced Parkinson's disease, according to a Thursday news release from Fridley, MN-based Medtronic.
The Activa PC+S DBS system delivers DBS therapy while also sensing and recording electrical signals in key areas of the brain, using sensing technology and an adjustable stimulation algorithm. The idea is that the system will provide key insights for researchers.
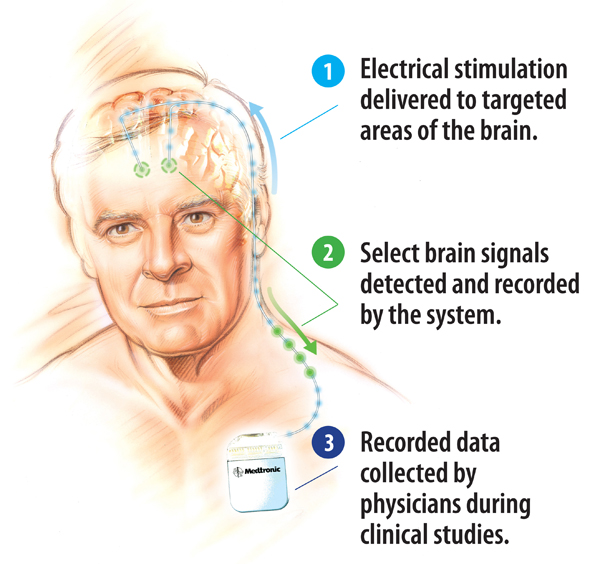
A Medtronic graphic shows how the Activa PC+S DBS system works.
Hopefully the data will inform the creation of future personalized DBS therapy in the same way that information collected from pacemakers informed pacemaker design.
"While DBS therapy is widely proven to treat symptoms of advanced Parkinson's disease and other movement disorders, the ability to collect and analyze data demonstrating how the brain responds to this therapy was not possible until now," says Philip Starr, MD, a professor of neurological surgery at UCSF whose early research helped lead to first human uses of the Activa PC+S DBS system.
Deep brain stimulation is used as a treatment for Parkinson's disease, essential tremor, dystonia, and treatment-resistant obsessive-compulsive disorder, with Medtronic DBS therapy benefitting more than 80,000 patients worldwide since the late 1990s.
A patient's viral YouTube video from earlier this year provided a visually-striking example of how deep brain stimulation therapy can mitigate the symptoms of Parkinson's disease. A man named Andrew Johnson turned his tremors on and off using a remote control for the DBS device.
The FDA has not yet approved the the Activa PC+S DBS for commercial use, but has allowed it to be implanted for research purposes. The system received CE Mark approval in January, with the first ever implant taking place at Ludwig Maximilian University in Munich, Germany, in August.





