Richard Burgess Les Miller and David VanLangevelde, Kaydon Bearings Division
Corrosion is the natural enemy of a rolling element bearing, and a sneaky enemy at that. When chemical reactions from moisture, vapors, and acids attack bearing surfaces, the damage they cause often isn't apparent until it's too late. Fortunately, there are many things you can do at the design stage to protect your bearing from these sneak attacks.
The first step, as usual, is to completely define the service conditions. The second is to conduct your review of the available techniques of corrosion protection with these requirements in mind. You may find advice from a bearing manufacturer or lubricant producer helpful at this early stage, as they typically have experience in a wide range of applications.

Service Conditions
There are many service conditions to consider, but they all fall into 5 main categories: performance requirements, contaminants, corrosion resistance ratings, frequency of maintenance, and extreme conditions.
1. Performance requirements
Many performance criteria should be considered. The most common are:
What loads must the bearing support? What is the expected duty cycle? What type of rotation is required, and at what speed? What degree of positioning accuracy? How long must it last, e.g. L(10) fatigue life? How will it be mounted? What lubrication is needed? How accessible will it be for maintenance?
These performance criteria are not typically affected by a corrosive operating environment, but you may find it necessary to make a few design modifications to assure that every one of them is met. The nature of the modifications will depend in large part on the nature of the contaminant(s).
2. Identify contaminants
Most contaminants are some sort of atmospheric moisture, but they take different forms and can have different concentrations. Water is the most obvious, but even water isn't simple: the same protection isn't effective against both ion-rich liquid and oxygen-rich condensates, for example. Salts and acids offer many more variations; if the bearing will be exposed to one of them it is important to know which type, and its concentration.
It's a good idea to look beyond contaminants and consider the effects of every element in the operating environment. Some are obvious (extreme temperatures and pressure), but others are not. Under some circumstances, for example, dry lubricants will react with water to produce sulfuric acid in the bearing race.
Bearings are also exposed to contamination before installation, during manufacturing, shipping, and storage. Moisture-proof packaging is a good idea throughout all these stages. In addition, facilities that apply corrosion-resistant coatings tend to have acidic atmospheres, so any uncoated bearings produced there should be protected with a lubricant film.
3. Corrosion resistance ratings
Bearings should have a corrosion life about the same as their expected service life. Why, for example, choose a material that offers 20 years of corrosion protection (like 17-4 precipitation hardening stainless steel) for a bearing with an expected fatigue life of only 6 years? Conversely, you can't expect a fatigue life of 10,000 hours from a shipboard bearing made of materials likely to rust solid after being exposed to salt spray for just 96 hours. All criteria must be in sync.
4. Frequency of maintenance
Corrosion resistance ratings are specific to a bearing's environment, and maintenance is a prime factor in any environment. To some extent, anti-corrosion reliability is a shared responsibility involving the designer, the machine operator and, in many applications, the technician who removes the bearing for inspection or service. In settings that are only mildly corrosive, proper maintenance performed on a diligently followed schedule may be sufficient — assuming, of course, that the bearing is accessible. Regardless, knowing how often the bearing will be removed will help you determine how much anti-corrosion reliability must be built into the design.
5. Extreme environments
While all corrosive environments are hard on bearings, some are especially extreme. Outer space is a good example. For the Phoenix Mars Lander that NASA launched in 2008 to collect soil and ice samples, a key requirement was to operate smoothly at -108°C, some 171 million miles from the nearest service technician. The bearings had to be heated and packed with a low-outgassing lubricant that wouldn't become too viscous in such extreme temperatures or evaporate in the thin atmosphere.
Corrosion Protection Options
Once all the service conditions have been defined, you can start assessing your bearing protection options and narrow your search accordingly. Lubricants, seals, coatings, and alternate materials are the most common forms.
Lubricants: While a lubricant film's primary function is to minimize metal-on-metal wear (and sometimes to cool the bearing), some lubricants can double as protection in a mildly corrosive environment. A highly water-resistant grease — such as a MIL-PRF-23827, like Shell's Aeroshell 7 — can help protect a bearing in a slightly damp environment while other greases cannot.
This protection, of course, is only possible when the lubricant film is intact. Should it overheat, dry out, or settle between operating cycles, it cannot be relied upon. Some people try to compensate by over-lubricating, but this simply increases the frictional torque and usually causes the bearing to overheat. Idle equipment should be rotated periodically to maintain the lubricant's protective properties.
Seals: Seals are commonly used to keep particles out of a bearing (and lubricant in). But typical seal materials (nitrile rubber, PTFE) do not provide much protection against liquids or gases, and those are the forms corrosion usually takes. You'll get better results making the bearing of a corrosion-resistant material, or a material that will accept a corrosion-resistant coating.
Protective coatings: Various protective coatings are used on bearing components, from plating and zinc thermal spray to electro-deposited and chemical-deposited barriers.
Chromium and nickel plating offer excellent corrosion resistance and are effective in highly corrosive environments, such as acid-bath plants or salt-spray exposures. Baked-on, solid-film lubricants or phosphates of zinc and manganese give good resistance and are suitable for moderately corrosive operating conditions.
Preservative oil or black oxide coatings are suitable only for very short-term protection from mildly corrosive environments. They are sometimes used for storing bearings before service. Zinc thermal spray, which gives the metal cathodic protection, offers a longer-term solution.
Eventually, however, most platings and coatings begin to separate from the base metal — chipping, flaking, or peeling. One exception: electrodepositing high-density chromium on races of traditional 52100 bearing steel. Independent tests have shown that electro-deposited, high-density chromium — such as the Endurakote® process used by Kaydon Bearings — resists corrosion at least as well as 440C stainless steel.
These protective coatings are applied more often to the bearing rings than the rolling elements, whose shape and comparatively small diameter make it difficult to keep even 0.0001-in. thick plate within allowable tolerances. For rolling elements, a material change is usually advisable.
Alternate materials: In extremely corrosive environments, the best approach — and often the most costly and most time-consuming — is to substitute corrosion-resistant materials for standard steel. Martensitic stainless steel and ceramic are standard materials today for rolling elements, and races can also be made of ceramic.
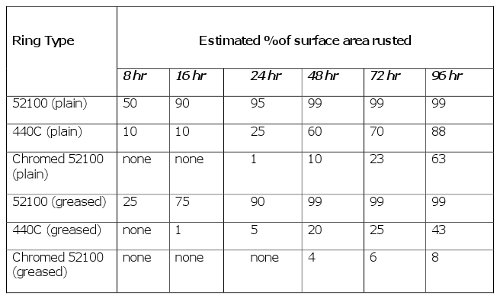
Salt Spray Resistance Tests
Tests by Kaydon Bearings Division demonstrate that electro-deposited chromium plating is an extremely effective barrier against corrosion from salt spray.
Twelve bearing race rings were divided into 2 sets, each set containing 6 rings — 2 each of plain 52100 steel, 440C stainless steel, and 52100 coated with electro-deposited, high-density chromium. Researchers treated one ring of each material with a water-resistant grease meeting the MIL-PRF-23827 specification and left the other ring of each material untreated.
The races were suspended in a test chamber and subjected for 96 hours to 5% sodium chloride spray at 95°F. The researchers inspected the rings periodically and recorded the progress of corrosion. The averaged results of the two tests are:?
Conclusion
Corrosion can have a serious impact on bearing life, which in turn affects a machine's performance, productivity, and cost of operation. Design engineers can minimize this impact by considering all of the bearing's operating conditions when setting the initial design criteria, and including one or more corrosion protection alternatives in their specifications. For assistance comparing the options or evaluating them for a particular application, please contact the experienced application engineering staff at Kaydon Bearings.





