The EnterMedics Maestro Rechargeable System weight loss treatment device is effective although scientists are unclear why.
The FDA has approved the first weight-loss device since 2007, giving obese adults an alternative to dieting and bariatric surgery.
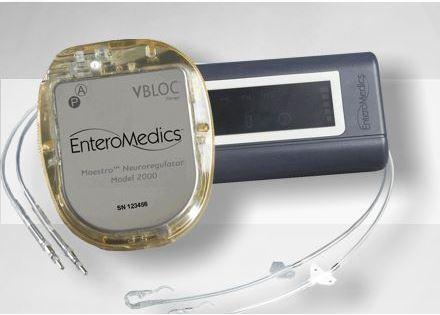
The Maestro Rechargeable System made by EnteroMedics Inc. (NASDAQ: ETRM), acts like a pacemaker that targets the nerve that runs between the brain and the stomach to control feelings of hunger and fullness.
The FDA approved the device to treat patients aged 18 and older who have been unable to lose weight with a traditional weight-loss program, who have a body mass index of 35 to 45, and at least one other obesity-related condition, such as type 2 diabetes, according to a statement by the agency.
The Maestro VBLOC system consists of a rechargeable electrical pulse generator, wire leads and electrodes that are implanted surgically into the abdomen. It sends intermittent electrical pulses to the trunks in the abdominal vagus nerve, which regulates stomach emptying and signals the brain that the stomach feels empty or full, the FDA statement said.
Neuromodulation enhances or suppresses activity of the nervous system by delivering electrical, chemical, or other agents to reversibly modify brain and nerve cell activity, according to the International Neuromodulation Society. More common uses of neuromodulation include spinal cord therapy to treat chronic pain, deep brain stimulation for essential tremor, Parkinson’s disease, Tourette syndrome, dystonia, epilepsy, and some psychiatric disorders, the organization’s website says.
External controllers would allow patients to charge the Maestro device and healthcare professionals to adjust its settings for optimal therapy with minimal side effects, the FDA said.
In a yearlong clinical trial of 233 patients with a BMI of 35 or greater, Roseville, MN–based EnteroMedics found that about half of the group of157 using the device as designed (the experimental group) lost at least 20% of their excess weight. Another 38.3% of patients in the same group lost at least 25% of theirs. The experimental group lost 8.5% more excess weight than the control group of 76 patients, whose devices were implanted but not activated, the FDA said.
The agency approved the Maestro device despite a shortcoming in the clinical trial and some mystery behind how the device works.
The experimental group failed to meet the clinical study’s goal of losing at least 10% more excess weight than the control group. However, an FDA advisory committee (the Gastroenterology and Urology Devices Panel) found that 18-month data showed sustained weight loss, and agreed that the device’s benefits outweighed the risks.
Serious side effects reported in the trial included nausea, pain at the neuroregulator site, vomiting, and surgical complications. Other adverse events included pain, heartburn, problems swallowing, belching, mild nausea, and chest pain.
The FDA also admitted that it does not know exactly how the Maestro works. “The specific mechanisms for weight loss due to use of the device are unknown,” the agency’s statement said.
Perhaps the dangers of obesity outweighed the mystery. According to the Centers for Disease Control and Prevention, more than one-third of all U.S. adults are obese, and people with obesity are at increased risk of heart disease, stroke, type 2 diabetes, and certain kinds of cancer.
EnteroMedics also holds a patent for an obesity treatment method that down-regulates nutrient absorption in a patient’s small intestine. EnteroMedics’ stock closed Wednesday at $1.40 per share, up more than 18% in heavy trading.





