The world of two-dimensional (2-D) materials has just gotten a little more crowded. If graphene, boron nitride, molybendum disulfide and silicene weren’t quite enough, we now may have something to join the mix in the 2-D universe that will go by the name “borophene.”
In experiments and simulations based at Brown University, in Providence, R.I., researchers took a big step toward a long theorized material made up of single-atom sheets of boron. The researchers haven’t actually produced borophene, but they did make a needed precursor structure that proves that the material is possible.
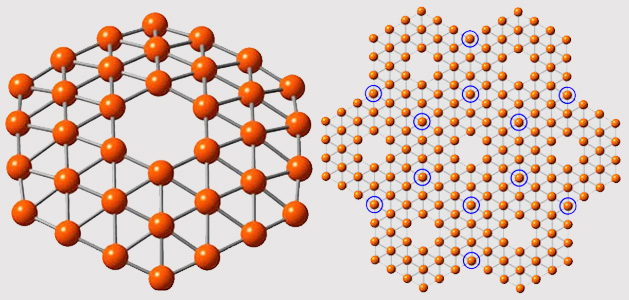
In research that was published in the journal Nature Communications (“Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets”) a cluster of 36 boron atoms were formed into a structure that resembles theoretical predictions for borphene: a symmetrical, one-atom thick disc with a perfect hexagonal hole in the middle.
The prediction was that boron, which has one fewer electron than carbon, wouldn’t be able to form into the honeycomb-lattice pattern that graphene takes. Instead it would likely form into a triangular lattice and a hexagonal hole would form in the middle of the sheet. That prediction pretty closely resembles what the researchers were able to produce in the lab.
"It’s beautiful,” said Lai-Sheng Wang, professor of chemistry at Brown, in a press release. “It has exact hexagonal symmetry with the hexagonal hole we were looking for. The hole is of real significance here. It suggests that this theoretical calculation about a boron planar structure might be right.”
Producing the boron structure was not anything like using the “Scotch Tape” method for producing graphene. The researchers were able to make the cluster of 36 boron atoms by first shooting a laser at bulk boron to vaporize it into boron atoms. They then shot the vapor of boron atoms with a jet of helium that froze the atoms into clusters. After being frozen, they were zapped again with a laser. This second laser kicks an electron out, which is funneled down a tube of sorts. By measuring the speed of the electron, they can determine how strongly the cluster holds onto its electrons, which is known as the electron binding energy spectrum. This spectrum is distinct, like a fingerprint, to the cluster’s structure.
Based on this binding energy spectrum, which indicated that it was very low energy compared to other boron clusters, certain structures were possible—3000 possible structures in fact. By using a supercomputer the researchers could go through each possibility. When Wang saw that one of the possibilities was a planar disc with the hexagonal hole, he knew that was the structure to investigate.
The researchers plowed on investigating all 3000 structures with a supercomputer and finally concluded that the B36 structure was the one that most closely matched the spectrum measured in the physical experiments.
While theoretically borophene should have even more interesting electronic characteristics than graphene, it’s not clear from this work whether anyone will ever be able to make it.
Image: Wang Lab/Brown University





