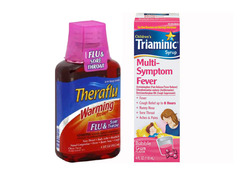
Novartis Consumer Health has recalled 2.3 million containers of Triaminic and Theraflu Warming Relief syrups because the child-resistant caps can be removed by children with the tamper-evident seal still in place, posing a poisoning risk.
The company has received 12 reports of children unscrewing the locked caps, including four reports of children ingesting the product. One child required medical attention.
The recalled products contain acetaminophen and diphenhydramine, which are required by the Poison Prevention Packaging Act to be sealed with child-resistant packaging.
The recall covers 24 types of Triaminic and Theraflu Warming Relief syrups. They were sold for about $5 nationwide at food, drug, mass-merchandise, and club stores between May 2010 and December 2011.
You'll find a complete list of products, lot numbers, and National Drug Codes (NDC) at www.novartisOTC.com. Lot numbers are on the bottom panel of the box and on the left side of the bottle label. The NDC number is on the upper-right corner of the front panel of the Triaminic syrups box and the upper-left corner of the Theraflu Warming Relief syrups bottle.
Find out how to prevent and treat the flu.





