The Direxion Torqueable Microcatheter-a microcatheter for shrinking tumors or blocking aneurysms-has won FDA and CE Mark approval, Boston Scientific recently announced.
The FDA granted the Direxion a 510(k) clearance because it retains the basic design of a previous Boston Scientific product, the Renegade Hi-Flo Microcatheter.
The catheters are for a technique called peripheral embolization in which interventional radiologists purposefully block a blood vessel to prevent blood flow to an area of the body. The procedure is used to treat liver cancer, uterine fibroids, and other challenging conditions.
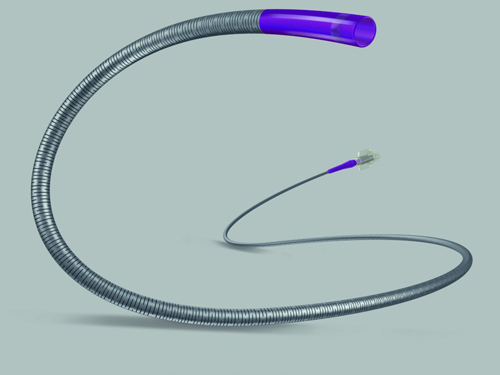
Boston Scientific officials think the Direxion is an improvement in a number of ways: It is designed to maximize torque transmission, and provide better handling during the procedure. It has six tip configurations and pre-loaded configurations designed to suit a range of peripheral embolization procedures.
"The Direxion Torqueable Microcatheter adds a completely new technology to our market-leading peripheral embolization portfolio, and its unique slotted nitinol hypotube technology will provide physicians with unrivaled handling characteristics," says Jeff Mirviss, president of peripheral interventions at Boston Scientific.
The Direxion also includes preloaded guidewires for physician convenience, which also required a change in packaging design, according to the FDA.
The Direxion's approval provides a sign that Natick, MA-based Boston Scientific continues to get new products approved by regulators, even as it has engaged in a string of restructurings and layoffs..
The company this week also announced that its Deep Brain Stimulation System has received CE Mark approval for the treatment of a neurological movement disorder called intractable primary and secondary dystonia.
Boston Scientific has also landed a CE Mark for its Lotus transcatheter aortic valve replacement (TAVR) device, which gives physicians a higher level of control than other devices on the market.
Despite a solid third quarter, Boston Scientific recently announced that it is slashing at least 1100 positions around the world. Total layoffs could be as high as 1500. The firm announced a similar number of layoffs earlier this year.





