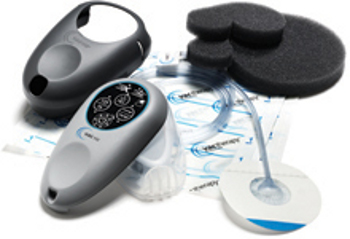
Kinetic Concepts (KCI) has obtained the US Food and Drug Administration (FDA) approval for its next generation V.A.C.Via negative pressure wound therapy system.
The V.A.C.Via therapy system is a single-patient-use, ultra-portable V.A.C. therapy system that provides simplified wound care for patients with moderate- to low-exudating wounds.
KCI has enhanced the original device to make it both effective and convenient for patients.
The enhancements include a diaphragm pump for faster draw down, longer battery life and higher leak rate.
In addition to providing all the benefits of V.A.C. therapy, V.A.C.Via therapy patients can enjoy V.A.C. therapy with a discreet, portable device; clinicians can enjoy off-the-shelf accessibility with single-patient-use application; and hospitals can enjoy greater cost savings with decreased length-of-stay.
KCI Americas senior vice president Jim Cunniff noted the company has listened to the feedback from its customers and patients and have taken the original system and enhanced it to better meet their needs.
"The V.A.C.Via Therapy Unit is designed to help patients return to their normal lifestyle, while receiving up to seven days of negative pressure wound therapy in a compact, portable unit," Cunniff added.
The system can be used in the acute setting for patients transitioning home and who are in need of continued V.A.C. therapy.
V.A.C.Via therapy system will be available in the US in early December 2013.





