SynGen has received 510(k) approval from the US Food and Drug Administration (FDA) to market its SynGenX-1000 System, CryoPRO-2 Cryopreservation/Storage Bag Set, SynGen DataTrak software products to process cord blood.
Previously, the company received ISO 13485:2003 certification and a Full Quality Assurance certificate from the British Standards Institution (BSI), which permits it to CE Mark its own Class 1 products and commercialize them in the EU.
SynGen CEO Philip Coelho said that the company is dedicated to developing products that consistently exceed our customers' expectations.
"We thank the FDA for guiding us through the 510(k) clearance process," Coelho added.
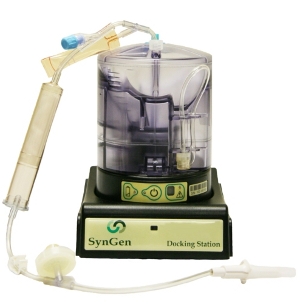
The SynGenX-1000 System features a programmable control module, docking station and disposable cartridge for harvesting stem and progenitor cells from units of collected cord blood. These cells are then used to reconstitute the hematopoietic system of patients suffering with hematologic malignancies such as leukemia and lymphoma and more than 70 genetic diseases.
The CryoPRO-2 Cryopreservation/Storage Bag Set is a disposable processing set that comes with a chamber into which the harvested stem and progenitor cells from the SynGenXTM-1000 are transferred, and then mixed with cryoprotectant and later put under sterile conditions into a multiple compartment bag for freezing and storage at liquid nitrogen temperatures of -196o C.
An application code, SynGen DataTrak software offers an interface between the operator and the SynGenX-1000 System through a host computer. From the SynGenX-1000 Control Module, it downloads processing and system information and later stores it in a database on the host computer.
Image: SynGenX-1000 System. Photo: Courtesy of PRNewswire/SynGen.