Royal Philips has secured 510(k) approval from the US Food and Drug Administration (FDA) to market its AlluraClarity live image guidance system.
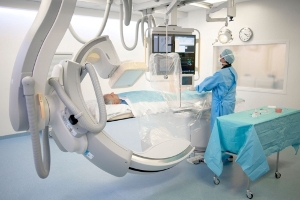
The company's AlluraClarity system, equipped with ClarityIQ technology, offers quality imaging for a wide range of clinical procedures, achieving greater visibility even when X-ray dose levels are low.
The Netherlands-located St. Antonius Hospital interventional radiologist Dr. Marco van Strijen said, "All patients treated via X-ray guided interventions benefit from the advantage of low radiation exposure, but it is especially important when you are treating patients who have to undergo lengthy and complex procedures."
Philips Healthcare Imaging Systems CEO Gene Saragnese said, "The transition from highly invasive surgical procedures to minimally-invasive image-guided therapies, with all their intrinsic patient benefits, is a transformation in the delivery of healthcare that is rapidly accelerating around the globe."
Commercially launched outside the US in 2012, more than 200 AlluraClarity systems have been ordered so far.
Image: Philips AlluraClarity interventional X-ray system. Photo: Courtesy of Royal Philips.





