For many suffering from blindness, the perfect Christmas gift has arrived. Next month, the Argus II Retinal Prothesis System by Second Sight is slated to go on sale in the United States.
The Argus II by Second Sight can restore sight for those suffering from retinitis. After landing FDA approval with a Humanitarian Device Exemption in February of this year, the device has gained a significant amount of media attention for the unique way that it can restore a patient's vision.
In the back of a normal human eye, the retina is filled with cones and rods that convert photons into electrochemical signals. These electrochemical impulses are transmitted through a person's optic nerve. However, some eye diseases like retinitis pigmentosa can impact this system, preventing the photoreceptors in the eye from working properly. Without functioning photoreceptors, the visual system in the eye can not transform light into imagery for the brain.
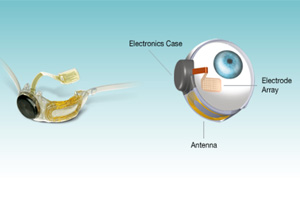
The Argus II device bypasses damaged receptors through the use of a "bionic eye." Like Google Glass, the Argus II includes a miniature camera attached to a specialized pair of glasses. Images captured by this camera are then transmitted to a video processing unit. Once this is done, this digital imagery data is transferred into a series of instructions.
Through the use of a wireless antenna, these instructions are transmitted to an implant inside a patient. This implant includes an electrode array that transmits pulses of electricity. These pulses can stimulate remaining cells in a patient's retina, allowing the transmission of visual data to a patient's brain. While the system can't provide the same level of vision as a normal, healthy eye, it can allow patients to perceive patterns of light.
Since digital technologies evolve at a rapid rate, the Argus II includes upgradeable components. Over time, cameras and visual processing units with higher resolution capabilities will give patients the ability to improve their sight.
While qualified patients will receive the device starting next month, the FDA approval comes with a significant caveat:
HUMANITARIAN DEVICE: Authorized by Federal (U.S.) law to provide electrical stimulation of the retina to induce visual perception in blind patients with severe to profound retinitis pigmentosa and bare light or no light perception in both eyes. The effectiveness of this device for this use has not been demonstrated.





