Wireless micro-LED devices are allowing researchers to use optogenetics to uncover information about molecular and cellular events in the brain that underlie stress, addiction and depression.
To better understand and one day provide improved treatments for depression, addiction and anxiety, researchers at Washington University School of Medicine in St. Louis are using tiny, electronic devices to identify and map neural circuits in the brain.
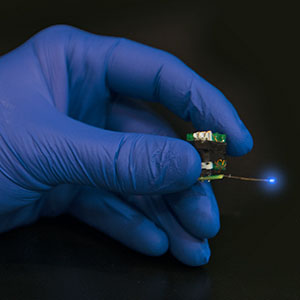
Wireless micro-LED devices are allowing researchers to use optogenetics to uncover information about molecular and cellular events in the brain that underlie stress, addiction and depression. (Photo Courtesy of Robert Boston)
The innovative work has been recognized with a rare grant called Exceptional, Unconventional Research Enabling Knowledge Acceleration (EUREKA) that funds high-risk/high-reward projects. The National Institutes of Health (NIH) supports 12 to 18 such grants each year.
With the award, Michael Bruchas, PhD, Assistant Professor of Anesthesiology, and his colleagues will conduct studies with micro-LED devices that his group recently co-developed with a team at the University of Illinois in Urbana-Champaign.
The work is part of the developing field of optogenetics, which uses advances in optics and genetics to control individual brain cells. For example, scientists can take a light-activated gene targeted at a particular type of neuron and insert the gene into a mouse. It then becomes possible to shine light into the animal’s brain either to get neurons to fire or to inhibit their activity.

Michael Bruchas, PhD, in the lab.(Photo Courtesy of Robert Boston)
In a recent study, Bruchas and his colleagues used the tiny electronic devices, which are thinner than a human hair, to tap into the internal reward system of mice, prodding their neurons to release dopamine, a chemical associated with pleasure, when the mice poked their noses through a hole in a particular part of a maze.
“Optogenetics allows us to zero in on specific populations of neurons and understand which ones are involved in complex behaviors,” Bruchas said. “What we learn from these studies will make it possible for us to target specific populations of brain cells that malfunction in depression, pain, addiction and other disorders.”
The four-year, US$ 1.2 million grant will permit Bruchas and his team to develop specialized, optically sensitive G-protein-coupled receptors on brain cells that will make it possible to control cell signaling in the brain with light. Combining these new receptor tools with wireless micro-LED devices implanted in the mouse brain should allow the researchers to uncover more information about molecular and cellular events that underlie stress, addiction and depression.
For example, Bruchas hopes to isolate and map the brain networks involved in stress by studying how mice interact in their cages. A dominant mouse may limit a cagemate from moving about freely, creating stress that may be apparent in the mouse’s brain networks.
Although many scientists use optogenetic techniques to isolate pathways in the mouse brain, often those animals are tethered to wires. Bruchas and his colleagues have the advantage of studying animals that are able to move freely because the LED devices that he and his colleagues have developed are portable and wireless. With funding from the EUREKA grant, his team plans to make them even smaller and add sensing capabilities to measure dynamic changes in the brain’s chemical transmission.





