Tangent Medical, a manufacturer of IV therapy products, has commercially launched its NovaCath Integrated IV Catheter System and commenced its clinical evaluations in several US healthcare centres.
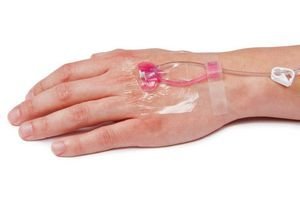
Considered to be the first safety IV catheter, NovaCath provides advanced catheter stabilization technology and exceeds several standards for IV catheter stabilization including CDC, OSHA and INS.
The NovaCath Integrated IV Catheter System, which has a passive needle shielding technology and closed system design, can minimize the occupational exposure to blood to the lowest possible extent.
Tangent Medical CEO Jeff Williams said the NovaCath Integrated IV Catheter System was developed after a year of clinical immersion to address many patient and clinician needs, while providing benefits to healthcare facilities and hospitals in the area of compliance and economics.
"The anticipated launch of the NovaCath has resulted in significant clinical interest and demand for the product," Williams added.
Equipped with ergonomic design and soft materials, the latest product can provide more comfort to patients and minimize the risk of pressure ulcers that can be due to the use of conventional catheters.
Moreover, the product has a 180° fluid-path turn that removes the J-Loop, which can lead to IV tubing snags and catheter dislodgement.
Due to its pre-assembled, all-in-one system, the IV catheter set-up time and risk of touch-point contamination gets minimized.
Tangent Medical Sales & Marketing vice president Curtis Bloch said that the clinical feedback supports NovaCath's ability to reduce overall IV complications while improving clinical efficiency and healthcare worker safety.
"No other catheter on the market combines advanced stabilization, passive needle encapsulation, tubing management and blood control on every single start," Bloch added.
Image: The NovaCath(TM) Integrated IV Catheter System. Photo: Courtesy of Market Wired.





