White light
There are two primary ways of producing white light-emitting diodes (WLEDs), LEDs that generate high-intensity white light. One is to use individual LEDs that emit three primary colors— red, green, and blue — and then mix all the colors to form white light. The other is to use a phosphor material to convert monochromatic light from a blue or UV LED to broad-spectrum white light, much in the same way a fluorescent light bulb works.
Due to metamerism, it is possible to have quite different spectra that appear white.
RGB systems
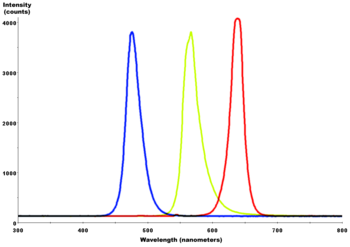
Combined spectral curves for blue, yellow-green, and high-brightness red solid-state semiconductor LEDs. FWHM spectral bandwidth is approximately 24–27 nm for all three colors.
White light can be formed by mixing differently colored lights; the most common method is to use red, green, and blue (RGB). Hence the method is called multi-color white LEDs (sometimes referred to as RGB LEDs). Because these need electronic circuits to control the blending and diffusion of different colors, and because the individual color LEDs typically have slightly different emission patterns (leading to variation of the color depending on direction) even if they are made as a single unit, these are seldom used to produce white lighting. Nevertheless, this method is particularly interesting in many uses because of the flexibility of mixing different colors, and, in principle, this mechanism also has higher quantum efficiency in producing white light.
There are several types of multi-color white LEDs: di-, tri-, and tetrachromatic white LEDs. Several key factors that play among these different methods, include color stability, color rendering capability, and luminous efficacy. Often, higher efficiency will mean lower color rendering, presenting a trade-off between the luminous efficiency and color rendering. For example, the dichromatic white LEDs have the best luminous efficacy (120 lm/W), but the lowest color rendering capability. However, although tetrachromatic white LEDs have excellent color rendering capability, they often have poor luminous efficiency. Trichromatic white LEDs are in between, having both good luminous efficacy (>70 lm/W) and fair color rendering capability.
One of the challenges is the development of more efficient green LEDs. The theoretical maximum for green LEDs is 683 lumens per watt but today few green LEDs exceed even 100 lumens per watt. The blue and red LEDs get closer to their theoretical limits.
Multi-color LEDs offer not merely another means to form white light but a new means to form light of different colors. Most perceivable colors can be formed by mixing different amounts of three primary colors. This allows precise dynamic color control. As more effort is devoted to investigating this method, multi-color LEDs should have profound influence on the fundamental method that we use to produce and control light color. However, before this type of LED can play a role on the market, several technical problems must be solved. These include that this type of LED's emission power decays exponentially with rising temperature, resulting in a substantial change in color stability. Such problems inhibit and may preclude industrial use. Thus, many new package designs aimed at solving this problem have been proposed and their results are now being reproduced by researchers and scientists.
Phosphor-based LEDs
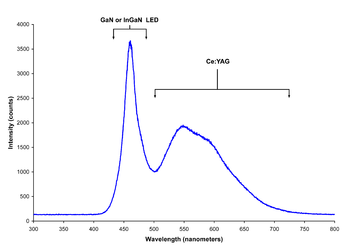
Spectrum of a “white” LED clearly showing blue light directly emitted by the GaN-based LED (peak at about 465 nm) and the more broadband Stokes-shifted light emitted by the Ce3+:YAG phosphor, which emits at roughly 500–700 nm.
This method involves coating LEDs of one color (mostly blue LEDs made of InGaN) with phosphors of different colors to form white light; the resultant LEDs are called phosphor-based white LEDs. A fraction of the blue light undergoes the Stokes shift being transformed from shorter wavelengths to longer. Depending on the color of the original LED, phosphors of different colors can be employed. If several phosphor layers of distinct colors are applied, the emitted spectrum is broadened, effectively raising the color rendering index (CRI) value of a given LED.
Phosphor-based LED efficiency losses are due to the heat loss from the Stokes shift and also other phosphor-related degradation issues. Their efficiencies compared to normal LEDs depend on the spectral distribution of the resultant light output and the original wavelength of the LED itself. For example, the efficiency of a typical YAG yellow phosphor based white LED ranges from 3 to 5 times the efficiency of the original blue LED because of the greater luminous efficacy of yellow compared to blue light. Due to the simplicity of manufacturing the phosphor method is still the most popular method for making high-intensity white LEDs. The design and production of a light source or light fixture using a monochrome emitter with phosphor conversion is simpler and cheaper than a complex RGB system, and the majority of high-intensity white LEDs presently on the market are manufactured using phosphor light conversion.
Among the challenges being faced to improve the efficiency of LED-based white light sources is the development of more efficient phosphors. Today the most efficient yellow phosphor is still the YAG phosphor, with less than 10% Stoke shift loss. Losses attributable to internal optical losses due to re-absorption in the LED chip and in the LED packaging itself account typically for another 10% to 30% of efficiency loss. Currently, in the area of phosphor LED development, much effort is being spent on optimizing these devices to higher light output and higher operation temperatures. For instance, the efficiency can be raised by adapting better package design or by using a more suitable type of phosphor. Conformal coating process is frequently used to address the issue of varying phosphor thickness.
The phosphor-based white LEDs encapsulate InGaN blue LEDs inside phosphor coated epoxy. A common yellow phosphor material is cerium-doped yttrium aluminium garnet (Ce3+:YAG).
White LEDs can also be made by coating near-ultraviolet (NUV) LEDs with a mixture of high-efficiency europium-based phosphors that emit red and blue, plus copper and aluminium-doped zinc sulfide (ZnS:Cu, Al) that emits green. This is a method analogous to the way fluorescent lamps work. This method is less efficient than blue LEDs with YAG:Ce phosphor, as the Stokes shift is larger, so more energy is converted to heat, but yields light with better spectral characteristics, which render color better. Due to the higher radiative output of the ultraviolet LEDs than of the blue ones, both methods offer comparable brightness. A concern is that UV light may leak from a malfunctioning light source and cause harm to human eyes or skin.
Other white LEDs
Another method used to produce experimental white light LEDs used no phosphors at all and was based on homoepitaxially grown zinc selenide (ZnSe) on a ZnSe substrate that simultaneously emitted blue light from its active region and yellow light from the substrate.





