Posted in Cardiovascular by Chris Newmarker on January 15, 2014
Boston Scientific CEO Mike Mahoney remains optimistic about renal denervation and the company’s Vessix platform, even though Medtronic’s Symplicity system failed on efficacy in a pivotal U.S. clinical trial.
The Vessix, acquired in 2012 for an upfront payment of $125 million (up to $425 million if milestones are met), is more advanced than the Simplicity, Mahoney told analysts at the J.P. Morgan Healthcare Conference on Tuesday.
“We are excited about the Vessix platform, potentially still in hypertension but also for other diseases. … We think we acquired it at a very good price,” Mahoney says.
Renal denervation involves applying radiofrequency ablation through a catheter to the renal artery that supplies the kidneys with blood. The minimally invasive procedure is supposed to lower blood pressure by cutting off nerve signals to the artery.
Mahoney touted the Vessix as balloon-expandable device, with multi-point capability where electrodes turn on and turn off.
The Vessix has generated some buzz over its iPad-inspired graphic user interface. The materials used in the device also reveal the influence of consumer technology.
“We think there’s ease of use benefits. There’s clearly speed benefits. We think it’s a better platform,” Mahoney says.
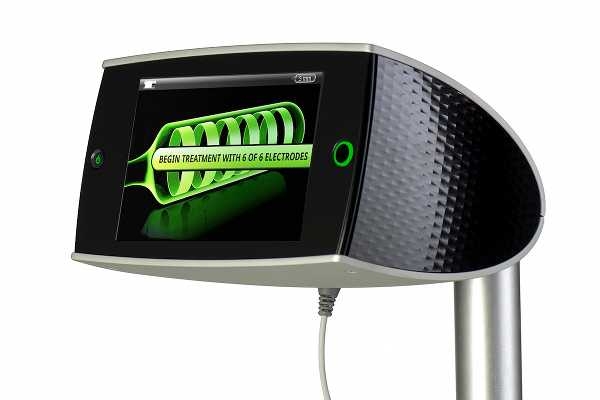
The Vessix has generated some buzz over its iPad-inspired graphic user interface. The materials used in the device also reveal the influence of consumer technology.
Clinical data from Europe, where the Vessix is approved, is “very good.” But Mahoney acknowledged that the Symplicity HTN-3 study results that Medtronic released last week were a surprise to the industry.
The Symplicity HTN-3 study, which involved a randomized 535 treatment-resistant hypertension patients in 87 U.S. medical centers, demonstrated that the treatment was safe. But it did not meet its goals for lowering blood pressure among those who received the treatment, versus the control group that underwent a sham procedure. Those in the control group had the option to receive the treatment after the six month assessment was over.
Researchers had seen promise in the procedure, which has received significant media attention in recent years. But the Symplicity HTN-3 results are causing some reevaluation, with Medtronic suspending enrollment in renal denervation hypertension trials in the United States, Japan, and India.
Boston Scientific plans to take the Vessix to trial in the U.S., so the company wants to learn from the Medtronic trial, according to Mahoney.
“We’re going to make sure we take our time and really evaluate the clinical results of that trial and use that information as a basis for our next steps in determining what the best clinical strategy is,” Mahoney says.





