A novel microfluidics device by IBM could be a boon to the oncology market. The new device can assist oncologists in identifying different types of cancer, offering several improvements over existing biopsy techniques.
When a patient has a suspected cancerous growth, physicians often remove tissue from that region for further testing. This process, known as a biopsy, typically uses chemical markers that respond differently in the presence of cancerous and noncancerous cells. However, biopsy samples are usually very small in size. In difficult-to-diagnose cancer cases, a multitude of tests may be necessary for positive identification. However, biopsy samples are often too small for use in multiple tests.
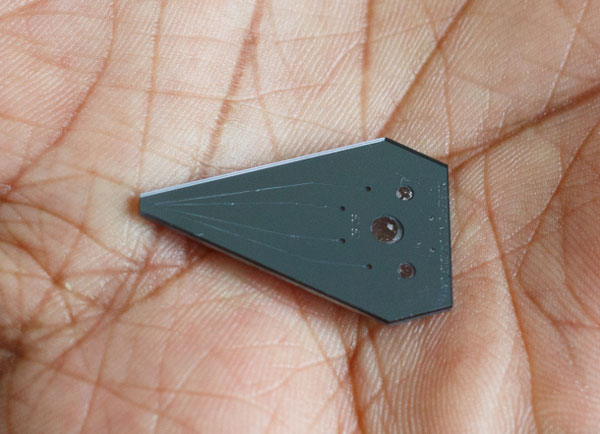
IBM's novel device can be used for improving cancer diagnosis times. The device works by pumping a miniscule amount of fluid into a biopsy sample on a plate. The device then removes excess fluid to prevent excessive staining. Image courtesy of CNET and Stephen Shankland.
To get around this problem, IBM developed a novel diagnostic chip that makes use of microfluidics. With this technology, researchers were able to shrink the total surface area required for biopsy testing to approximately 100 micrometers. To test a biopsy sample, the device pumps a marker chemical down a microfluidic sample. Once this is done, the marker fluid is sucked up a second channel. This technique can be used to prevent the chemical marker fluid from spreading outside of a designated biopsy patch. During this process, researchers place the sample under a microscope to observe changes that could indicate cancer.
Researchers believe the new technique could be an effective way to test biopsied tissues that are available in limited quantities. For example, a tissue sample of 1 square meter could theoretically be used for 500 tests. Even more astounding, these 500 tests would take place on approximately 5% of the biopsy patch.
Biopsies are widely used to diagnose cancer and to determine if a lesion is benign or malignant. The volume of tissue typically harvested in biopsies, however, is minimal, generally precluding that it be used in a variety of tests.
IBM researchers may have a breakthrough that enables more extensive testing of small tissue samples.
Since the tip channels for the microfluidics device are on the micrometer scale, they can be precisely placed to "inject" chemical markers in the right location. If the microfluidics device is loaded with several different marker reservoirs, researchers can quickly switch between different marker chemicals, allowing for a wide-spectrum test.
That said, researchers did note that the technology still faced some limitations. As of now, the tips for the microfluidics device are manually "steered" through the use of a precision joystick. For a high-count marker analysis, this could be a time-consuming effort. In the future, researchers home that automated technologies could guide the microfluidics device, allowing for higher testing numbers.
In addition to this, researchers hope that future versions of the device will be more robust. Since the tips on the device are smaller than a human hair, ensuring a durable build is essential for future versions of this device.





